Subsections of Equipment
Lateral stereo microscope illumination
One of the main problems in the preparation of gastrotriches is to track down the animals in a water sample and pipettate on an object carrier.Since gastrotriches are transparent, they are almost invisible in a stereo microscope with traditional incident or transmitted light illumination.The animals only become visible when the field lighting is dark.
In the professional area, dark field lighting is realized by cold light conductor. LED lights with swan neck offer an alternative option that illuminate the sample on the side.This lateral lighting only gets the light spread on the objects into the lens of the microscope.The background remains pure black.Since the lamps are not attached to the sample, the illuminance and the shadow throw changes depending on the sample position.Often the lamps also represent a mechanical obstacle when shifting the sample vessel. This type of lighting is usually not available in stereo microscopes and must be retrofitted.
In order to minimize these disadvantages, I have developed a side dark field lighting for this purpose, which can be easily realized with simple means and a 3D printer and can be adapted to any stereo microscope.
Holder for LED strips
Rotatable object guide
To align slides according to the DIC gradient or the image edges of the camera, it is necessary to rotate the slides on the stage. If you - like me - do not have a rotation stage at your disposal, you have to come up with something…
I have constructed a rotatable object guide, which can easily be manufactured with a simple 3D printer. The following properties were taken into account:
- low height - thus collisions with the objectives are excluded
- mounted to the stage, so that the specimens can still be moved
- specimen rests directly on the stage - no problem with focusing the condenser / Köhler is possible
- easy insertion and removal of the slide
- different dimensions of the slides possible due to spring holder
- insertion of the commercially available plastic Petri dishes with 7 cm diameter possible; additional circular recess for the use of the lids of the Petri dishes (whose height is somewhat smaller, so that no collision with the 10 objective can occur)
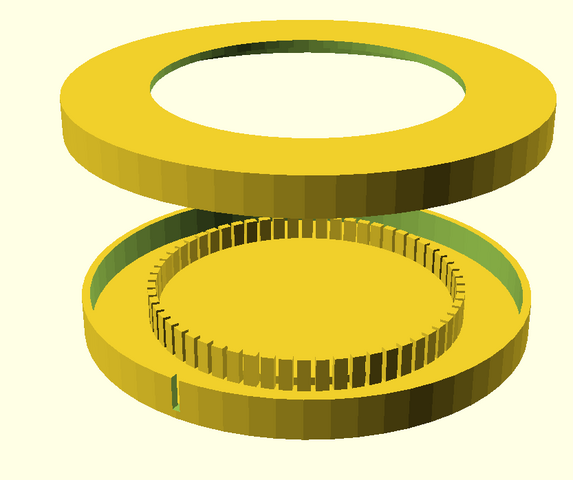
The object guide consists of two separate parts:
- a rotating frame which is fixed to the stage with the usual fixing screws
- a turntable which is placed in the frame and can be rotated in it. To facilitate the rotation, the turntable rests on the frame only at six points. The turntable is equipped with six handles so that it can be easily operated from all sides
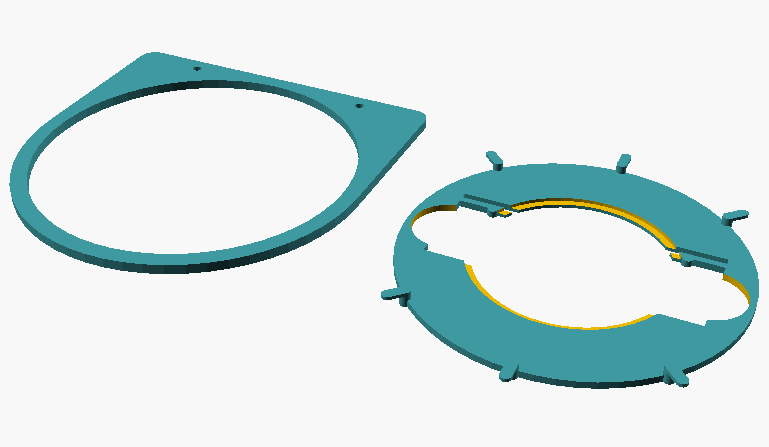
Fig. 1: Individual parts of the specimen guide; on the left, frame that is mounted to the stage; on the right, the turntable that holds the slide or Petri dish.
The two parts are inserted into each other. The slide sits firmly in the recess and can be rotated 360 degrees and oriented as desired. The round recesses are provided for Petri dishes, if necessary.
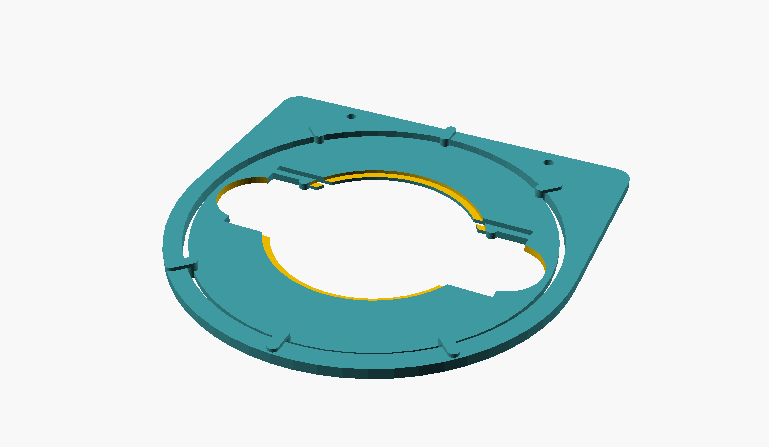
Fig. 2: assembled object guide
At the microscope the whole thing looks like this:

Fig. 3: Rotatable object guide on microscope
This design has proven itself in practice. Of course, the rotary stage cannot be centered when rotating, the position of the specimen must be readjusted by the stage. The slides fit tightly enough so that there is no “slippage” at all when moving them. However, some slide marks seem to have a slightly smaller depth, so one wishes they would sit a little tighter on those. If you want to use such slides, you have to adjust the retaining springs a bit….
The corresponding print files - including the CAD files in OpenSCad format can be downloaded from Thingiverse. The design is adapted to the stage of my Ortholux II. For other microscopes, the position or diameter of the holding screws may have to be adjusted.
I would not like to miss this practical object guide anymore. Only by rotating the object to the optimal angle is it possible to achieve, for example, optimal contrasting with DIK. Also the alignment at the photo edges saves the constant turning of the camera.
Flash Light
Dhe advantages and disadvantages of a microflash setup on the microscope have already been discussed in detail in several articles. For me as a “Enthusiast of the Pond”, the decisive factor was that a microflash
- allows to observe with minimal light intensity (of the pilot light). Unfortunately, there are many organisms that do not tolerate well the often high light levels required for DIC and panic to avoid the light or even do not survive the illumination.
- ensures a constant white balance of the photos independent of the light intensity of the pilot light.
- allows short exposure times independent of the pilot light. If one wants to realize short exposure times with the “normal” LED illumination, one typically has to set the lamp to maximum light intensity before releasing the shutter, but this is uncomfortable for observation. This is the only way to then achieve exposure times shorter than 1/500 s, which is necessary to “freeze” the motion blur.
- Motion blur minimized with typical exposition times of less than 1/2000 at ISO 100. With LED lighting, I typically achieve only 1/200 s at 100 DIK at ISO 400.
That’s why I constructed flash equipment for my Ortholux II with my 3D printer!
With a microflash, in principle, you have the fairly simple option of mirroring the flash to the side under the condenser. However, such a setup is rather provisional, since during “normal operation” the flash and beam splitter under the object stage interfere and limit the ergonomic operation. For a permanently attached microflash on a microscope with a separate lamp house, the “flash cubes” have proven themselves. They are inserted between the lamp house and the stand and here reflect the flash into the “normal” beam path. This also allows flash illumination under “Köhler condition”, since the existing light field diaphragm is also used for the flash. So it had to become a similar flash cube solution, where the necessary mechanical components should be created with a 3D printer. My solution looks like this:
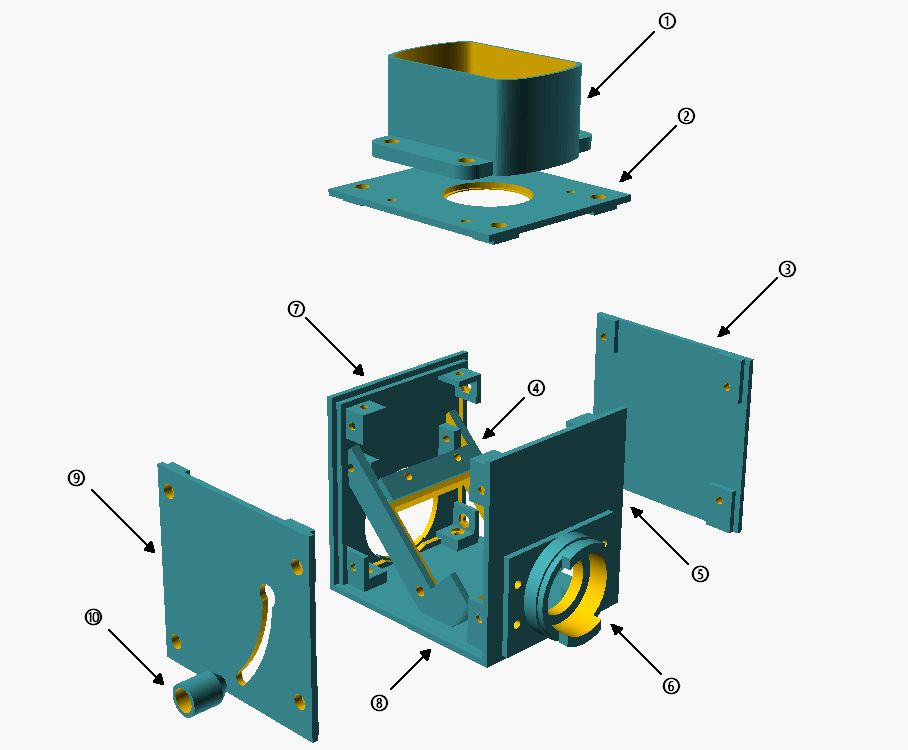
Exploded view of flash cube: 1: flash holder; 2: cover; 3: side wall; 4: rotating beam splitter holder; 5: wall side; 6: lamp bayonet microscope side; 7: lamp bayonet lamp side; 8: bottom plate; 9: side wall with swivel handle; 10: spring-loaded handle.
The central component is the beam splitter holder (4), which would be designed to swivel. This makes it possible to remove the beam splitter from the beam path and also to operate the illumination beam path without interference from the flash. To ensure a secure hold of the swung-out beam splitter, the swivel handle (10) was also attached to the swivel axis with a spring, so that it presses against the side wall and locks into the recess in the upper position. To swivel in the beam splitter, the handle must be pulled outward to allow free movement. On the lamp and stand side, the cube walls (6 and 7) were designed according to the dimensions for the lamp bayonet on the Ortholux II. This allows the flash cube to be firmly mounted between the lamp and stand and does not further interfere with the operation of the microscope. The light aperture (2) on the lid of the flash cube has been adapted to the dimensions of the collector lens so that it can simply be inserted directly. Therefore, when transporting, the collector lens should be removed, as it is held in place only by gravity. Due to this construction, the flash sits directly on the collector lens and the distance to the flash tube is almost optimal. The Fresnel plate of the flash cannot cause any interference in the image, as it sits very close to the collector lens and is therefore not in focus. A holder (1) for the flash is screwed onto the top, which is of course adapted to the dimensions of the flash unit used.
Assembled, the whole thing looks like this:
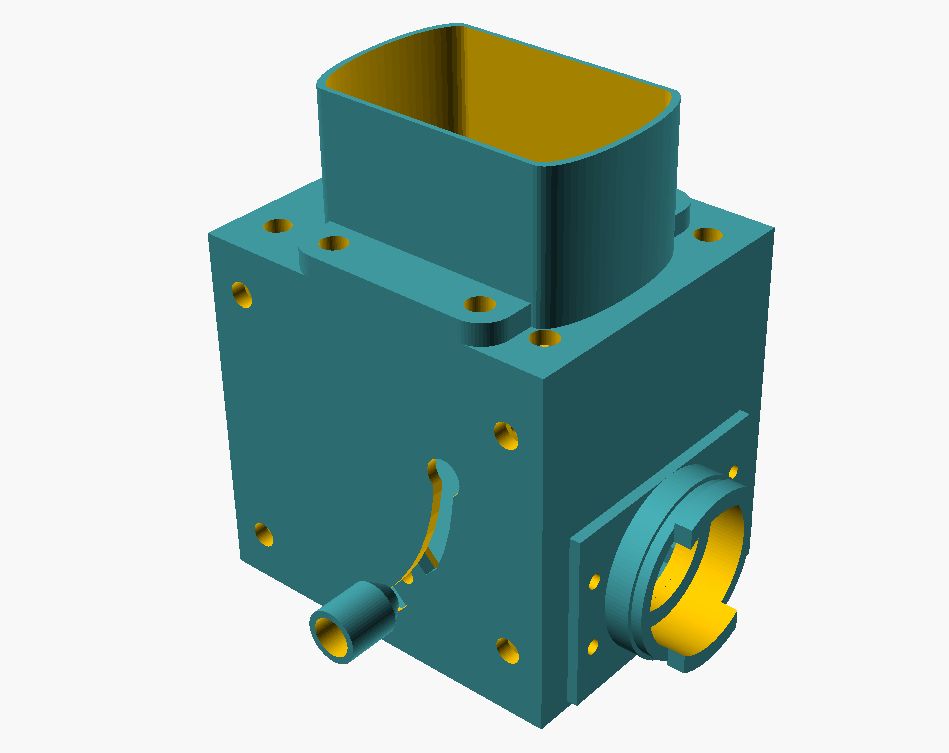
overall view
The outer dimensions (without flash holder) of the flash cube are 90mm * 100mm * 105mm - so it is actually a “flash cuboid”. These dimensions are due to the dimensions of the purchased parts (beam splitter, collector lens, flash) and the specifications of the lamp bayonet. In reality at the microscope, the microflash device looks like this:

Flash on Ortholux II
As a flash I use a Godox TT685II for Canon cameras with wireless remote control, which works without problems with my EOS700D. The flash has a guide number of 60 and thus enough power to still expose correctly with 1/8 of the maximum power at the 100 DIC. I operate the flash in TTL mode, which easily adjusts the flash power to the subject / lens. Only an exposure compensation of -1.7 EV had to be set. This exposure compensation is independent of the lens used, but sometimes needs to be adjusted to the subject - just like with normal photos. To operate the flash with a power supply, I replaced the batteries with battery dummies, to which a 6V power supply is connected. For those who want to try a replica, I have put together the corresponding OpenSCAD files here for download. An adaptation to the geometry of other beam splitters, collector lenses and flash units is possible without any problems. More difficult is the adaptation to lamp bayonet microscope manufacturers. This is possible in principle, but may require changes to the beam delivery, as the position of the light axis may then change. If you have problems, just ask - everything is possible somehow…
I am very satisfied with this microflash solution and would not want to miss it anymore. Unfortunately, this eliminates another excuse for my photo quality - now I guess I have to learn how to take good photos after all…