Aspidiophorus squamulosus
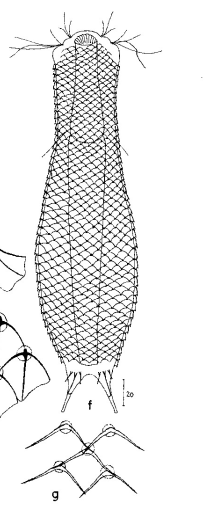
115 µm - 265 µm
Width:
30 µm - 42 µm
Width of the head ( five-lobed ):
20 µm - 28 µm
Width of the neck:
17 µm - 26 µm
Length of the furca:
15 µm - 30 µm
Adhessive tubes:
50% of furca
Pharyx ( cylindrical, terminal swollen ):
23 µm - 45 µm
Diameter of the mouth ( around ):
12 µm
Dorsal scales:
12-15 rows of 40-42 petiolar scales each with rhombic terminal plates without median keel (3-4 x 5-6 µm); scale shell ends in anal region; posterior end and toes naked; 3 pairs of short spines at base of toes (8-10 µm).
Ventral scales:
2 terminal keels (5-6 µm), 12 rows of keels without base plates
Oecology:
Mud dweller
Similar species:
A. paradoxus : unkeeled basal plates; spination at the posterior end; mouth grafting
Particularities:
Mouth armament pointed clasp
Fundorte:
Of the (acc. (Schwank, 1990) ) twenty limnic Aspidiophorus species only four are listed in the “official” species lists for Germany. Another species was described some time ago by Michael Plewka. The species Aspidiophorus squamulosus has been found so far only in Poland and in western France - so it is no wonder that this animal is also native to us, between the previous finding areas.

Fig. 1: Aspidiophorus squamulosus; optical section and scale image.
The internal structure of the animal becomes quite clear in the side view:

Fig. 2: A. squamulosus, side view.
A. squamulosus is with ca. 230µm somewhat smaller than the similar species A. paradoxus and is clearly distinguished by the three spines each above the toes (pic. 3, ob. li. and mi. r.). In addition, the terminal plates of the petiolar scales do not possess a median ridge. Also the prominent, furrowed hypostomium (Fig. 3, ob. r.) is typical for this species. Some animals I found were in their “hermaphroditic phase” (post-parthenogenetic phase) and showed a very large X-organ and sperm bundle (Fig. 3, mi. l.). One animal also carried a very large egg (with a pronounced nucleolus), which it laid during the approximately 10-day observation period. Unfortunately, I was not able to observe the oviposition itself (also the egg was not found in the specimen).

Figure 3: A. squamulosus: details;
up. l.: toe spines; up. r.: hypostomium
mi. l.: X-organ and spermathecae (marking); mi. r.: toe spines and toe scales;
low. l.: transverse section of scale carapace; low. r.: stylet (mark)_
As is often the case, there were some deviations from the species description in the literature. With up to 50 generations per year and years of isolation in a small garden pond, I find minor deviations not surprising. However, it is possible that some details were not noticed in the rare, earlier observations. For example, the base of the toes should be unscaled (Fig. 3, mi. r.). However, the specimens I observed show many very small, roundish and close-scaled scales. Only the conspicuous peduncle scales do not appear at the posterior end of the animal.
In some Aspidiophorus species movable “teeth” in the mouth tube are described. Although A. squamulosus should be toothless, two actively movable styletes could be observed, the tip of which ended in the mouth tube and reminded of tardigrades (Fig. 3, un. r.).
The peculiar stipe scales of the genus Aspidiophorus are not to be grasped in their three-dimensional beauty in the scale association. Therefore, I subjected a pitiable specimen of A. squamulosus to scale analysis:

Fig. 4: A. squamulosus; colored and - unfortunately - flattened single scales.
Since during a scale analysis just the very small scales are pressed rather flat, I tried a drawing reconstruction of the three-dimensional structure:
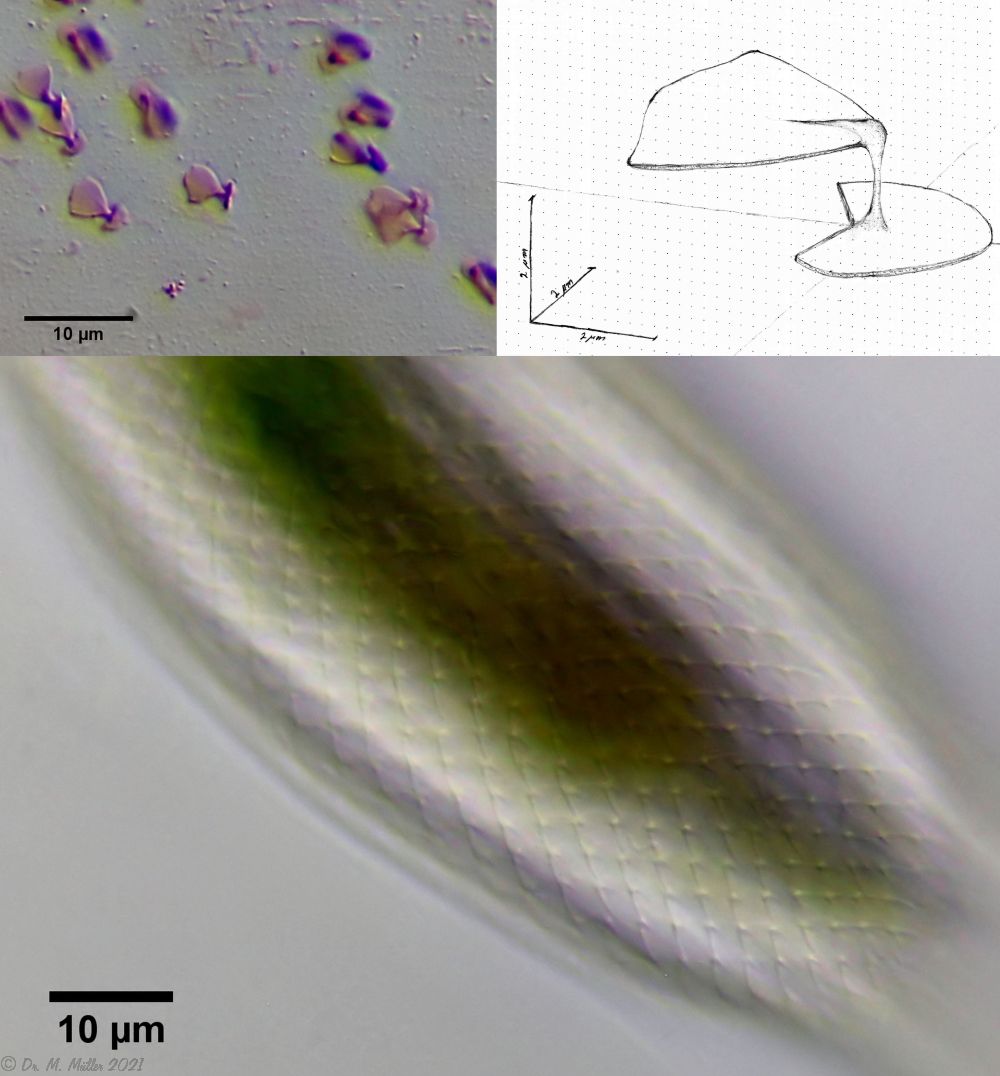
Fig. 5: A. squamulosus; bottom: composite scale image; top left: isolated and stained scales; top right: drawing reconstruction of a single peduncle scale.
Another point that has remained open so far is the diet of A. squamulosus. The main diet consists of relatively large unicellular algae or eye flagellates. To swallow this large prey, the mouth of the animals is equipped with movable lamellae that can be folded outward for feeding (cf. Captochaetus). This allows the diameter of the mouth opening to be greatly expanded, unlike most gastrotrichs. During the swallowing movement, the stylet is pressed into the mouth tube and the prey cells are guided past it. In the process, the hard cell wall is probably perforated so that the digestive secretions in the intestine can penetrate the cells.

Fig. 6: A. squamulosus; intestine filled with ocular flagellates.
If one examines the mud of stagnant waters microscopically, one always finds gastrotrich clutches in empty shells of water fleas with sometimes dozens of eggs.

Fig. 7: A. squamulosus; focal sac of a gastrotrich clutch containing eggs of at least three different species.
Often these clutches consist of eggs of different species. Apparently, a number of species prefer to lay their eggs in water flea shells where other gastrotrichs have already laid their eggs.
In such a clutch often relatively large (95µm x 55 µm), spiny eggs are noticeable, which have not yet started embryonic development and probably represent permanent eggs.

Fig. 5: A. squamulosus; permanent egg.
One egg of the shown clutch started to develop and turned out to be an egg of the species A. sqamulosus. Therefore I can show here the eggs belonging to the species.
(Roszczak, 1935)